Select All the Intermolecular Forces Associated With 1-pentanol.
Explain why 1-propanol has a higher boiling point than 2-propanol. Select all the intermolecular forces associated with 1-pentanol.
Solved Select All The Intermolecular Forces Associated With Chegg Com
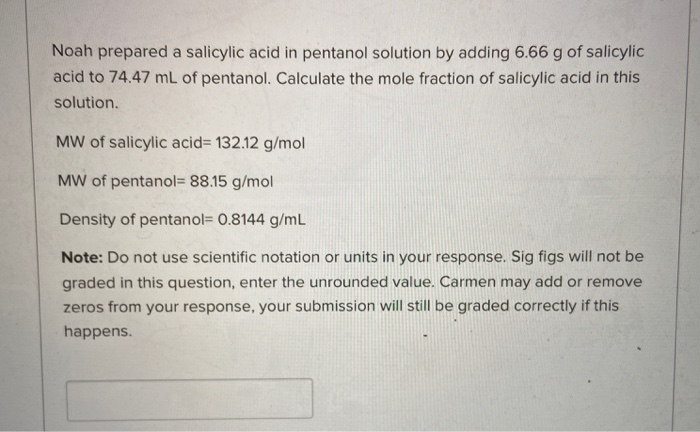
Calculate the mole fraction of salicylic acid in this solution.
. When evaporation takes place the heat comes from the. Which intermolecular forces do the following pairs of molecules experience. These forces mediate the interactions between individual molecules of a substance.
I think the cutoff is about 5 carbons - when you have n-pentanol this molecule is sparingly soluble in water even though it still has dipoledipole and H-Bondsthe London Dispersion Forces contribute more and the molecule ends up not liking water. Hydrogen bonds of the type OH-O between organics. Download the identifier in a file.
C 5 H 13 O 4 C 5 H 13 O 5 By formula. Intermolecular forces are responsible for most of the physical and chemical properties of matter. Compounds with stronger intermolecular forces larger masses and less branching will have higher boiling points.
C 5 H 12 O. Identify the intermolecular forces in CH4. A london dispersion B.
In both types of close packing74 of the total volume of the structure is occupied by the spheres and 26 is empty space. As the carbon chain gets longer the contribution of the London dispersion forces becomes significant. MW of salicylic acid13212 gmol MW of pentanol 8815 gmol Density of pentanol 08144 gmL Note.
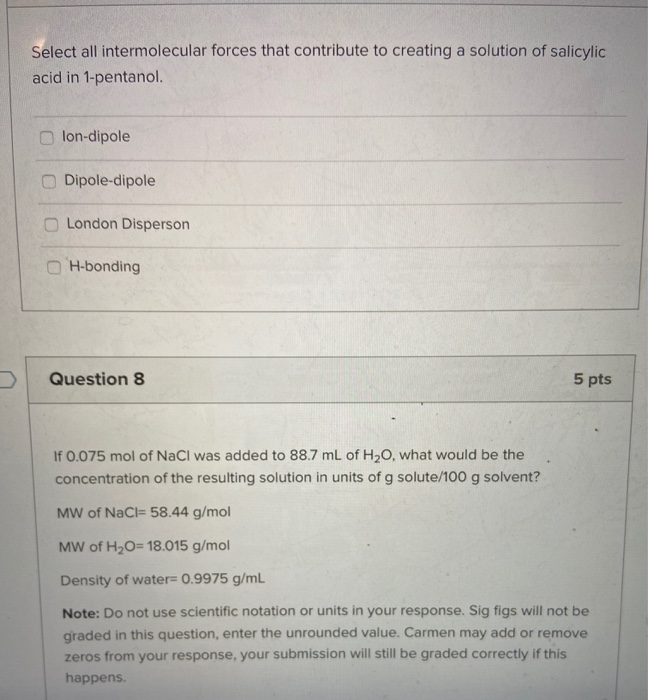
Evaporation requires the breaking of all intermolecular forces. It would take more energy to break the intermolecular forces in 1-pentanol. London Disperson Dipole-dipole lon-dipole H-bonding Select all intermolecular forces that contribute to creating a solution of salicylic acid in 1-pentanol.
London dispersion forces 1-Pentanol should have larger intermolecular forces due to H-bonding meaning the molecules are more attracted to each other than in pentane. Water molecules use hydrogen bonding to stick to each other not to from the bonds inside the water molecule itself. B H2O because it is capable of hydrogen bonding.
Now 1-propanol has a normal boiling point of 97 98 C. Compounds II and III only exhibit intermolecular London dispersion forces so they would be the two lowest boiling compounds weakest intermolecular forces. Pentane would better dissolve benzoic acid because like salicylic acid benzoic acid is actually a nonpolar hydrophobic compound due to its benzene group.
Select the compound that should have the lowest boiling point. For this reason it does not freely give up its proton to bond with water making it poorly soluble in water. The 1-Propanol can form London Force Dipole- Dipole and H- bonding due to the H bonded to O atom of OH group whereas the methoxyethane can not form the H-bonding.
In comparison each sphere in the body centered cubic structure has a coordination number of 8 and only 68 of the space is occupied. Noah prepared a salicylic acid in pentanol solution by adding 666 g of salicylic acid to 7447 mL of pentanol. Lon-dipole London Disperson H-bonding Dipole-dipole.
London Disperson Dipole-dipole lon-dipole H-bonding Select all intermolecular forces that contribute to creating a solution of salicylic acid in 1-pentanol. Do not use scientific notation or units in your response. Chemistry questions and answers.
These intermolecular forces allow molecules to pack together in the solid and liquid states. London Disperson H-bonding Dipole-dipole lon-dipole Select all the intermolecular forces associated with Naci. A chemist has three compounds of similar molecular weight but with different dominant intermolecular forces.
The snowflake falls yet lays not long Its feathry grasp on Mother Earth Ere Sun returns it to the vapors Whence it came Or to waters tumbling down the rocky slope. Their structures are shown below. Select all the intermolecular forces associated with 1-pentanol.
It must have H in the molecule. The intermolecular forces present in pentanol Determine the kinds of intermolecular forces that are present in. Pentanol with another molecule of pentanol.
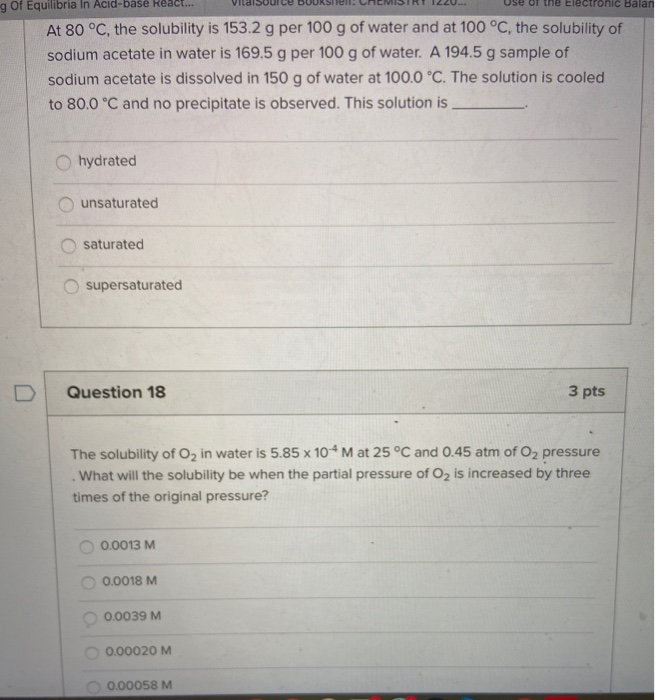
And we compare this to that of isopropanol 826 C and ethanol 780 C. Rank the following in order of increasing ability to form hydrogen bonds. Switching reactionThermochemical ladder CH33SiH2O Entropy change calculated or estimated.
Select all the intermolecular forces associated with H2O. The shape of snow flakes are actually determined by the shape of water molecules and the intermolecular forces between them. Intermolecular forces often abbreviated to IMF are the attractive and repulsive forces that arise between the molecules of a substance.
Predict the types of IM forces found in liquid. It is easier for 1-propanol to form hydrogen bonds than it is for 2-propanol. Requirements to be present.
AHCl because the dipole-dipole interactions of H-Cl better match the intermolecular forces of diethyl ether. Given these data there is another contributor to intermolecular interaction and here it is the non-polar interaction between hydrocarbyl chains. Up to 10 cash back Boiling point is highly dependent on the intermolecular forces of a compound.
Select the dominant intermolecular force of attraction between C5H12 molecules. The longer the chain the greater the chain-chain interaction and in. C CH3CH2MgBr because the non-polar ion interactions are more closely related to dipole-dipole than ionic bonding.
Identify the intermolecular forces in CH3Cl and CO. C 5 H 13 O 4C5H12O C5H12O C 5 H 13 O 5C5H12O Bond type.

Solved Select All The Intermolecular Forces Associated With Chegg Com

Solved Select All The Intermolecular Forces Associated With Chegg Com
Solved Select All The Intermolecular Forces Associated With Chegg Com
Comments
Post a Comment